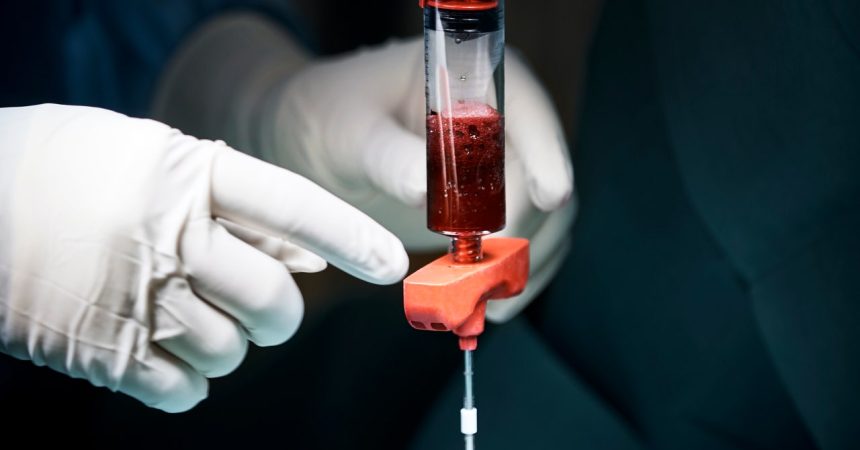
In discussions surrounding compensation for tissue donation, ethical considerations arise, particularly in the context of different country regulations. As noted by Bernow, individuals living on a modest monthly budget may find additional financial support from donations appealing; thus, a payment of $200 for donating stem cells could be beneficial. However, in the UK, legislation prohibits monetarily compensating donors for blood, plasma, or tissue, although they can receive reimbursements for incurred expenses. Meanwhile, in Sweden and several other EU nations, while donors can receive compensation for their time, outright payment is forbidden. This scenario underscores the complex balance between ethical practices and financial necessity, particularly for students or low-income individuals seeking additional income.
The question of whether donors should receive compensation sparks debate among scholars and ethicists. According to Hank Greely, a law professor at Stanford specializing in biosciences, the moral considerations surrounding compensation differ based on perspective. He categorizes the ethical dilemma as a “venial sin” rather than a severe moral failing, indicating a recognition of the practicality involved for individuals who may benefit financially from their contributions. In contrast, the United States allows for financial compensation in specific instances, such as sperm, egg, and blood donations, revealing a disparity between American and European approaches to tissue donation.
Stem cell donations uniquely impact the economics of medical treatments due to the substantial financial stakes involved in the medical industry. Bernow highlights that each donation can produce significant quantities of mesenchymal stem cells, which are crucial for therapeutic applications. With each donation yielding about 50ml of tissue and the potential to create around 200 treatment doses, the monetary implications are profound. For instance, one donation can equate to treatments worth over $3 million when factoring in the substantial costs associated with these medical procedures. In light of these figures, the $200 compensation for donors appears relatively nominal against the economic backdrop of stem cell treatment pricing.
Compounding this situation are the costs associated with preparing and distributing stem cell treatments. Bernow explains that while an intravenous treatment may cost around $25,000, only a portion of that is allocated to the donor’s compensation; much of the cost stems from the clinical trial infrastructure and the processes involved in handling the stem cells. Bernow’s startup aims to address these financial challenges by increasing the yield from each donation and minimizing the number of donors required. By leveraging economies of scale, the goal is to significantly reduce the cost of treatments within the next decade, illustrating an aspiration to make stem cell therapies more accessible.
However, Greely emphasizes that the paramount concern regarding stem cell treatments revolves around their efficacy and the potential misinformation circulating about these therapies. The U.S. Food and Drug Administration (FDA) has issued warnings about unapproved stem cell treatments that may be misleading or unsafe for patients. This suggests a critical need for regulatory oversight as many patients are drawn to unproven remedies that may pose significant risks. Consequently, the intricate web of financial compensation, ethical considerations, and regulatory frameworks reflects broader challenges within the evolving landscape of stem cell medicine.
In concluding, while financial compensation for stem cell donors can provide essential support for low-income individuals, the systemic implications of such practices necessitate careful scrutiny. The potential for significant profits from stem cell treatments—often at the expense of patient safety and regulatory diligence—raises vital ethical questions. Greely’s laissez-faire attitude towards donor compensation, framed within capitalism, highlights the complexity of balancing profit motives with ethical biomedical practices. As regulatory bodies and industry leaders navigate this landscape, ongoing discussions about compensation, ethical obligations, and safety will remain critical in shaping the future of stem cell therapies.