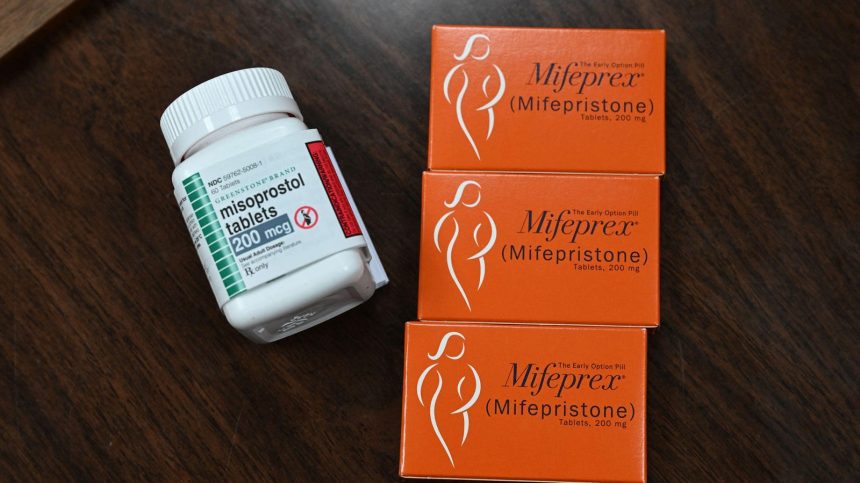
A recent proof-of-concept study has presented a potential alternative to the standard medication abortion regimen, offering a glimmer of hope amidst the ongoing legal battles surrounding reproductive healthcare access. The study, published in January 2025, found that ulipristal acetate, the active ingredient in the emergency contraceptive ella, combined with misoprostol, successfully terminated pregnancies in 97% of participants. This finding is significant as it suggests a viable substitute for mifepristone, the drug currently used in conjunction with misoprostol for medication abortions, which has become increasingly difficult to access due to legal restrictions and political challenges. The ulipristal-misoprostol regimen exhibited a high success rate with manageable side effects, offering a potentially safer and more accessible alternative for women seeking medication abortion.
The current standard for medication abortion involves a combination of mifepristone and misoprostol. Mifepristone blocks the hormone progesterone, essential for maintaining pregnancy, while misoprostol induces uterine contractions to expel the pregnancy tissue. However, the accessibility of mifepristone has been severely hampered by legal challenges and restrictions in several states following the overturning of Roe v. Wade. These restrictions include limiting the gestational age for mifepristone use, mandating multiple in-person doctor visits, and even classifying the drug as a controlled substance, effectively criminalizing its possession without a prescription. The escalating legal battles surrounding mifepristone underscore the urgent need for alternative medication abortion options.
Ulipristal, primarily known as an emergency contraceptive, functions differently from mifepristone. Emergency contraception prevents pregnancy by delaying or inhibiting ovulation, or by preventing implantation of a fertilized egg. It does not terminate an existing pregnancy. The study’s findings do not suggest that emergency contraception can be used as an abortifacient. Instead, the research demonstrates the efficacy of ulipristal, at a higher dose than found in emergency contraception, when combined with misoprostol for early medication abortion. This crucial distinction must be emphasized to avoid further misconceptions and potential restrictions on access to emergency contraception.
The potential benefits of using ulipristal as a replacement for mifepristone are multifaceted. Firstly, it offers a workaround to the legal obstacles surrounding mifepristone access, potentially ensuring that women can continue to access medication abortion even in states with restrictive laws. Secondly, ulipristal is already available in pharmacies, making it more readily accessible than mifepristone, which often requires a clinician’s prescription. Thirdly, ulipristal is less expensive than mifepristone, potentially reducing the financial burden on individuals seeking abortion care. These advantages position ulipristal as a promising alternative, particularly for women in underserved communities or regions with limited healthcare access.
However, the potential adoption of ulipristal for medication abortion also raises concerns. Public confusion regarding the distinction between emergency contraception and abortion pills could lead to misplaced efforts to restrict access to emergency contraception. Misinformation regarding the mechanism of action of emergency contraception, even amongst healthcare providers, could fuel these efforts. The high acceptability rating of the ulipristal-misoprostol regimen among study participants, coupled with its high success rate, underscores its potential as a safe and effective alternative. However, it is crucial to address the existing misconceptions surrounding emergency contraception and ensure its continued availability for women who rely on it for preventing unintended pregnancies.
The ongoing legal and political battles surrounding abortion access highlight the urgent need for accessible and affordable alternatives. The proof-of-concept study on ulipristal presents a promising avenue for expanding medication abortion options, particularly in the face of increasing restrictions on mifepristone. However, ensuring accurate information and dispelling misconceptions about emergency contraception is paramount to safeguarding access to this essential reproductive healthcare tool. The future of abortion access hinges on both scientific advancements and informed policy decisions that prioritize women’s health and reproductive autonomy. Further research is needed to solidify the findings of this initial study and to investigate the long-term safety and efficacy of the ulipristal-misoprostol regimen. Simultaneously, public education campaigns are essential to differentiate between emergency contraception and medication abortion, protecting access to both forms of reproductive healthcare.